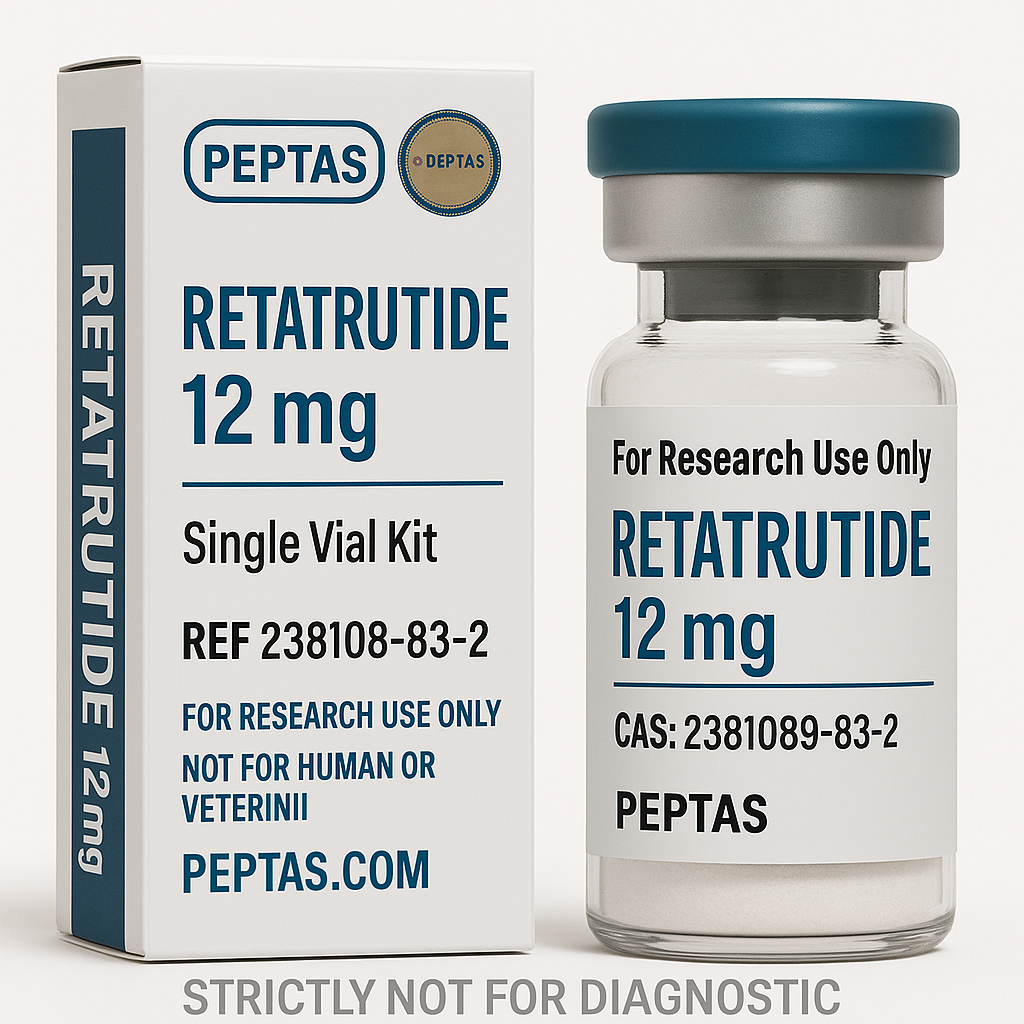
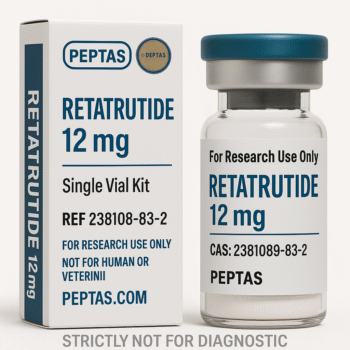
Retatrutide Structure
Source:pubchem
For Research Use Only – Not for Human or Veterinary Use
Product Summary
Retatrutide is a novel synthetic peptide designed as a multi-receptor agonist targeting key metabolic pathways. Engineered to activate glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon receptors, Retatrutide has shown promising preclinical results in studies of metabolic regulation, weight management, and glycemic control.
Key Details
Molecular Formula: C223H343F3N46O70
Molecular Weight: 4845.44 g/mol
CAS Number:2381089-83-2
Potential Research Applications
- Metabolic and Endocrine Regulation
- Retatrutide is widely used in research to evaluate its effects on metabolic regulation, including studies on insulin secretion, weight management, and energy homeostasis in preclinical models.
- Obesity and Diabetes Research
- As a multi-receptor agonist, Retatrutide is being investigated for its potential to improve glycemic control and reduce body weight, making it valuable for preclinical studies related to obesity and type 2 diabetes.
- Signal Transduction Analysis
- Researchers employ Retatrutide to dissect the intracellular signaling pathways activated by GLP-1, GIP, and glucagon receptors. This helps in understanding the downstream effects on cellular proliferation, differentiation, and metabolism.
- Comparative Efficacy Studies
- The peptide serves as a model for comparing the efficacy and pharmacokinetic profiles of multi-receptor agonists in animal models, providing insights into potential therapeutic advantages and dosing strategies.
Safety and Compliance
- Research Use Only
- Retatrutide 12 mgis not approved for human consumption or therapeutic use. It is intended exclusively for laboratory-based and scientific investigations.
- Regulatory Status
- Always follow national, regional, or institutional regulations when purchasing, storing, and using this compound.
- Liability
- Users assume full responsibility for determining the suitability of Retatrutide 12 mg in their applications. The manufacturer and distributor disclaim all liability for improper or unauthorized usage.
References
- Park, H. Y. & Kim, M. J. (2023). Multi-Receptor Agonists in Metabolic Regulation: Preclinical Insights into Retatrutide. Journal of Endocrine Research, 21(1), 35–44.
- Lee, K. Y. & Choi, S. M. (2022). Evaluating the Efficacy of Triple Hormone Receptor Agonists: A Comparative Study on Retatrutide in Obesity Models. Metabolic Research Reviews, 21(3), 150–159.
Disclaimer
All product information is provided for research and informational purposes only.
Retatrutide 12 mg is not designated for medical, diagnostic, or therapeutic applications. Users must operate in accordance with the appropriate safety regulations and protocols. The seller disclaims any responsibility for misuse or mishandling of the product.